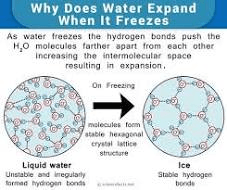
Why is frozen water (ice) less dense allowing it to float?
These are molecules have the same chemical formula but different structures
What are Isomers
The number of bonds that carbon can form.
What is 4
What functional group makes up water?
Hydroxyl (-OH)
Describe the pH scale. What is considered an acid, neutral and basic/alkaline.
What is 0-14. PH values less than 7 are acid, 7 is neutral and greater than 7 are basic/alkaline.
This is the name of the substance dissolved in a solvent.
What is Solute
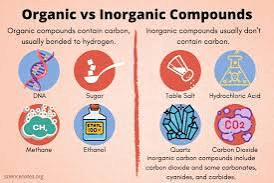
What is the difference between organic and inorganic molecules?
Organic molecules contain carbon and inorganic molecules do not contain carbon
Describe the biological levels of organization, small to large.
Atoms - molecules - macromolecules - organelles - cells - tissues - organs - organ systems - organisms - populations - communities - ecosystems - biosphere
This functional group is written as -COOH
What is Carboxyl
This is the name to describe the cohesive forces between water molecules that allows some insects to walk on water.
What is Surface Tension
What is the name given for water being attracted to other water molecules. What is the name for water being attracted to other molecules/substances?
What is cohesion? What is adhesion?
What are the 6 essential elements for life? These elements form the major biological molecules, nucleic acids, proteins, carbohydrates, and lipids.
What is carbon, hydrogen, oxygen, nitrogen, phosphorus and sulfur.
This is the study of microorganisms.
What is microbiology
This is the "amino" functional group
What is -NH2
A biomolecule's function is dictated by its _________.
What is structure
This is the heat required to change the temp of 1 gram of a substance 1 degree C.
What is Specific Heat? High specific heat = less fluctuation in water temperature
These molecules are mirror images of each other.
What isomers of Enantiomers
This is a blind process whereby scientists submit the details of their experiments, results and interpretations, and reviewers determine the veracity and merit of that work for publication.
What is a peer review
What functional group has -CH3?
This action is due to the pressure of cohesion and adhesion of water which cause the liquid to move up a tube.
What is Capillary Action
What is the difference between hydrophilic and hydrophobic substances?
Hydrophilic = water loving, can be dissolved in water, polar & ionic
Hydrophobic = water fearing/hating, cannot be dissolved in water, nonpolar
_________ bonds result from the attractive force between a hydrogen atom and a very electronegative atom such as N and O.
What is hydrogen bond
The absence of a membrane bound organelles is a characteristic of ________.
What is prokaryotes
What functional group acts like acids?
What is carboxyl group - COOH
What year did Mrs. Irby graduate high school?
What is 2000