Fe3+, Fe, Fe2+
Fe3+ <Fe2+ < Fe
The reasoning for why the lattice energy of MgO is greater than that of MgCl2
Mg and O have higher overall charges than Mg and Cl so the numerator of the Coloumb's law is greater and then energy holding them together is stronger
High temperature, low pressure
All of these molecules are similar in that they contain ___ IMFs
NH3
HF
H2O
H bonding
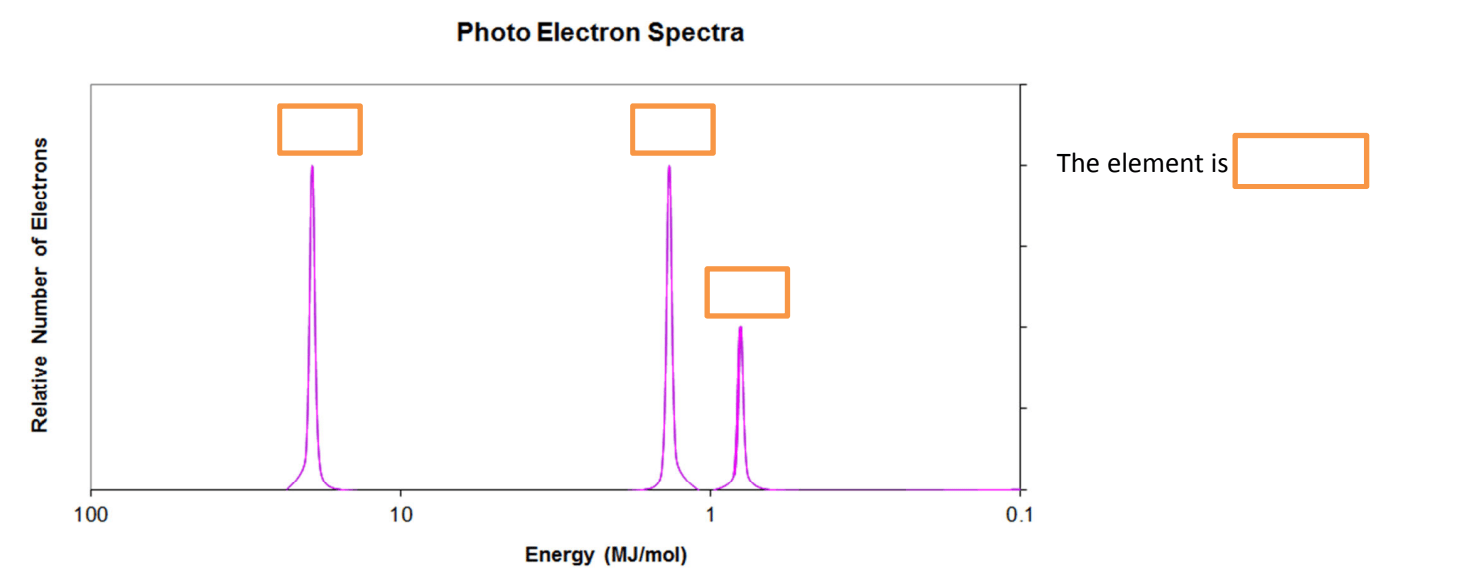
Boron
These atoms differ in molecular geometry because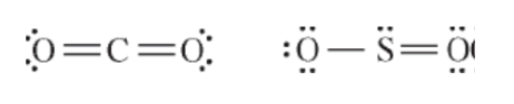
Sulfur has a lone pair on the oxygen which leads to a bent structure rather than a linear structure
How would this graph change if the temperature of the system was decreased
see board
Why is the boiling point of CO2 lower than that of SO2?
SO2 is polar and CO2 is nonpolar
True or False: The PES of Ca2+ and Ar should be the same
False
This is why ionic solids only conduct electricity in aqueous solution
Ionic solids separate into ions in solution surrounded by hydration spheres. This allows electrons (electricity) to move through the solution. In the solid state there is no motion or separate of ions so electricty can't conduct
These molecules will have the most similar rate of effusion
HCl and CO2
HCl and NH3
CO2 and SO2
HCl and CO2
An endothermic solution process has this relationship between the strength of IMFs formed and those broken
IMFs formed in the solution are weaker than those broken, Net gain of heat
This element is:
1s22s22p63s1
Sodium
True or False: The formal charges in this molecule represent a major contributor to the resonance structure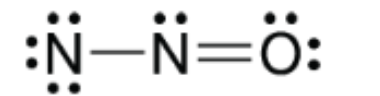
False
This gas has the highest average molecular speed when held at 500 K:
H2, O2, He, Ar
H2
Would H2O and NaCl form an ideal solution? Explain
No. Formation of ion-dipole interactions are more favorable than the interactions independently and therefore likely to be a negative deviation from Raoult's law
Mo
The hybridization of the carbon atom can best be described as
sp2
The impact that intermolecular forces would have on an ideal gas's behavior
Volume/Pressure
Using this phase diagram, explain why CO2 can sublime under our atmospheric conditions
asdf