What charges to protons, neutrons, and electrons each have
Protons - positive (+)
Neutrons - neutral (0)
Electrons - negative (-)
What is an element?
An element is a pure substance made of only ONE type of atom
What is a Molecule? Give an Example.
Two or more atoms bonded together.
Examples may vary.
How do you calculate the number of neutrons in an atom?
a. Same as the number of Protons
b. Atomic Number - Electrons
c. Atomic Number - Atomic Mass
d. Atomic Mass - Atomic Number
Atomic Mass - Atomic Number
What animal was hit by Mr. Schmid's car and then attacked him?
 Hawk
Hawk
Inside an atom there is mostly...... what?
Empty Space!
Potassium's Atomic Number is 19. Its Atomic Mass is 39.0983.
How many neutrons are in a Potassium Atom?
20 neutrons
This is a molecule of two hydrogen atoms (H2). What part of an atom determines how it bonds to others?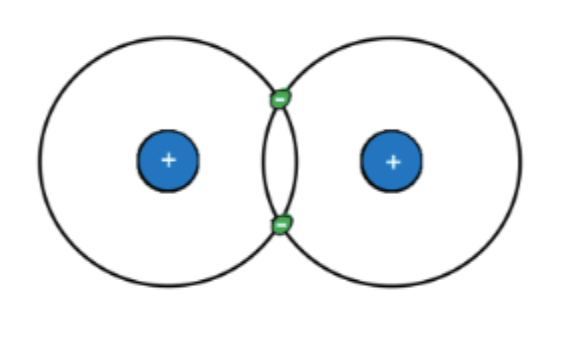
Electrons.
An atom's electrons (number, arrangment) determines how it will or won't bond with other atoms.
How many protons, electrons, and neutrons does Sodium have in its atoms?

11 protons
11 electrons
12 neutrons
Mr. Schmid lost his credit card one weekend. Before he could cancel the card, someone found it and purchased ____________ with Mr. Schmid's money.
a. Talkis
b. a car
c. a skateboard
d. a phone
Skateboard
Protons and Neutrons each weigh 1 AMU. How much do electrons weigh?
1/1800 - 1/1850
1/1836th (exact)
The weights of atoms, protons, neutrons, and electrons is measured in AMUs.
What does AMU stand for?
Atomic Mass Unit
This is a Carbon atom. How many more electrons does it need to have a full outer shell?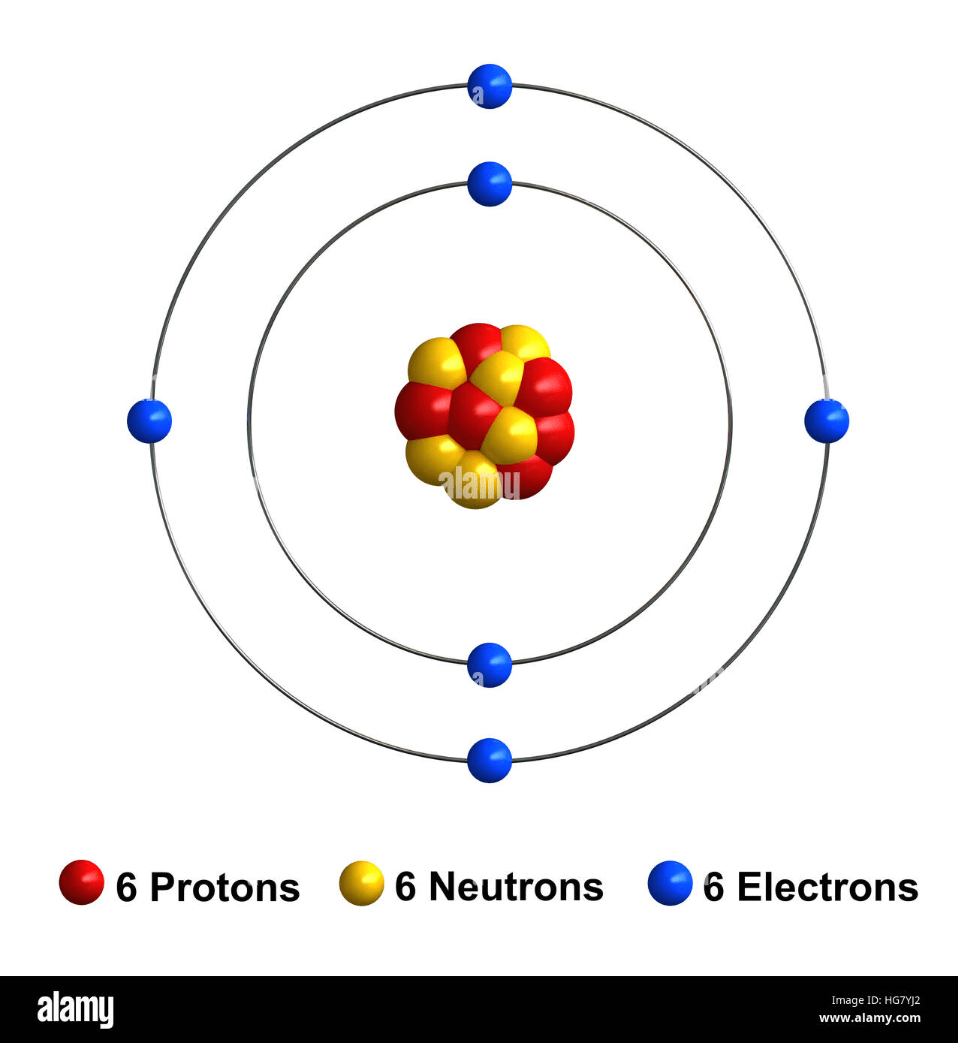
4 electrons
How many protons, electrons, and neutrons in a Zinc atom?
30 protons
30 electrons
35 neutrons
What sport did Mr. Schmid flunk out of in 6th grade?
a. Gymnastics
b. Baseball
c. Soccer
d. Tennis
Baseball
The word "atom" comes from the Ancient Greek ἄτομος (átomos), which means..... what?
Uncuttable
Indivisible
What does "synthetic" mean, and what is an example of a Synthetic element?
"Synthetic" means human-made.
Synthetic elements do not exist in nature.
Examples may vary.
This is an oxygen atom. How many bonds can it make with other atoms?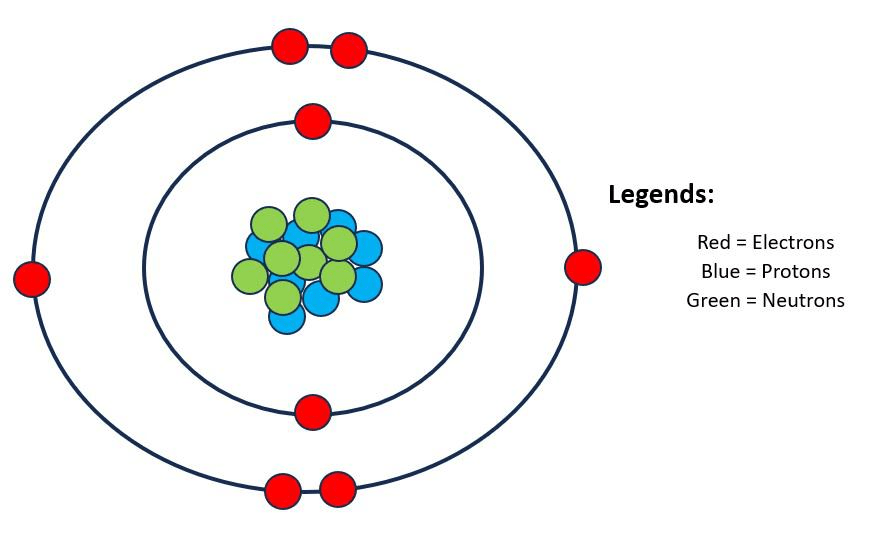
2 bonds.
(It needs 2 more electrons to have a complete outer shell of 8, so it can make 2 bonds)
What element is this?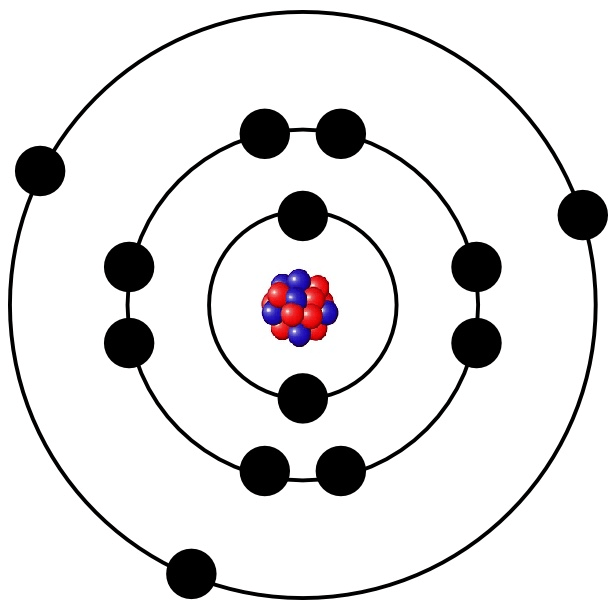
Aluminum
What two bones has Mr. Schmid broken?
a. Nose and Leg
b. Tailbone and Arm
c. Rib and Foot
d. Finger and Skull
Tailbone and Arm
What is an example of something NOT made of atoms?
Answers may vary.
Possible answers: Energy, Thoughts, Love, Light, Sound, Friendship, etc
These are atom from three Noble Gases - Helium, Neon, and Argon. Based on what you know about atomic structure, why are they called "noble" gases?
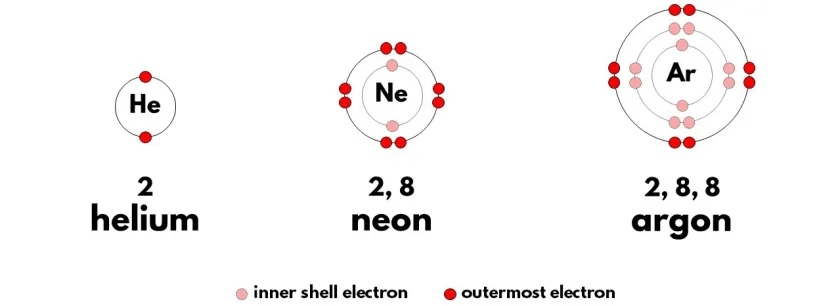
These elements are "Noble" because with full outer shells, they are not reactive and are stable, reliable, safe.
Aka Noble.
Which atom will be more reactive?
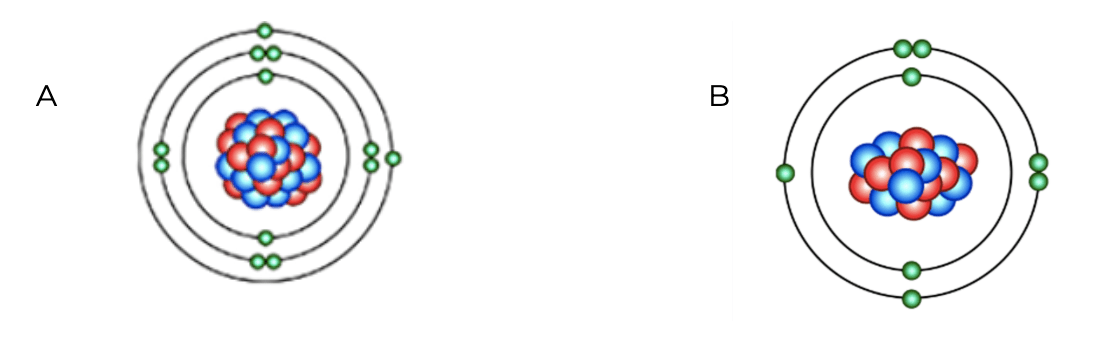
Atom A is more reactive.
This is because A's outer electron shell is almost empty. Atoms whose' outer shells are almost empty or almost full are more reactive.
What is an Ion?
An Ion is an atom that has missing or extra electrons.
An Ion is an atom that has a positive or negative electric charge.
What is the only animal that Mr. Schmid fears?
Leeches