The basic unit of matter is called what?
An atom.
What are valence electrons? Where are they found?
Valence electrons: electrons in the outermost energy level that participate in chemical bonding
Found in the energy level/shell
What is an ionic bond?
Bond that is formed between oppositely charged ions where they transfer electrons
What kind of structure is shown in the picture?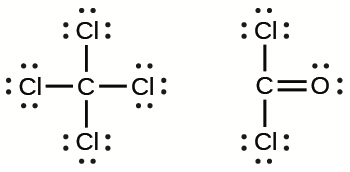
Lewis Structure: a diagram that represents/illustrates the bonds between atoms in a molecule and the lone pairs of electrons
The ________ of an atom holds the protons and neutrons.
Nucleus
What is the difference between a cation and an anion?
Cations are positively charged (since they lost electrons) while anions are negatively charged (since they gained electrons)
cation = +
What is a covalent bond?
Bond formed where the atoms share electrons
What kind of formula is shown below? (Molecular, Empirical, Structural?)
C6H12O6
Molecular
A molecular formula shows the number and types of atoms in a molecule
What three particles are found in atoms? Define them.
Proton: Positively charged particle found in the nucleus of an atom
Neutron: Neutrally charged particle found in the nucleus of an atom
Electron: Negatively charged particle that orbits the nucleus
T/F: Ions and molecules are the same thing
False.
An ion is an atom or molecule with a net electric charge due to loss or gain of electrons, i.e. an atom with a charge (ex. K+)
A molecule is a group of 2 or more atoms bonded together, which can be either the same or different elements (ex. H2O)
What is the difference between a polar and non-polar covalent bond?
Polar: type of covalent bond where electrons are shared unevenly, causing a molecule to have a partial charge (ex. H2O)
Non-polar: type of covalent bond where electrons are shared equally between atoms (ex. CO2)
What is an empirical formula?
A chemical formula that shows the simplest ratio of elements within a compound
ex. The molecular formula of glucose is C6H12O6. It's empirical formula would be CH2O since the ratio of elements is 1:2:1
What is the difference between the atomic number and the atomic mass/weight?
Atomic number: # of protons in the nucleus of an atom that defines the element
Atomic mass/weight: the average weight of the atom that accounts for isotopes
What does electronegativity mean?
A measure of an atom's ability to attract electrons in a bond.
In other words, the pull an atom has on electrons (ex. Oxygen has a higher electronegativity in H2O)
What is a hydrogen bond?
Weak bond between a hydrogen atom and an electronegative atom
1 way to remember: H likes to have FON (fluorine, oxygen, and nitrogen)
What kind of formula (Molecular, Empirical, or Structural) is shown in the picture below?
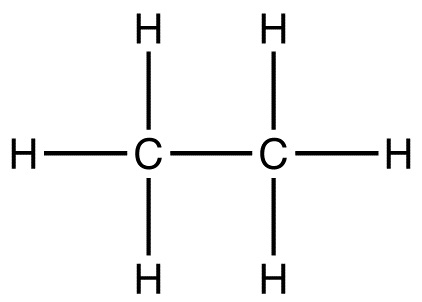
Structural
A structural formula shows the arrangement of atoms and the bonds between them in a molecule
What is an isotope?
What is an electron cloud?
Isotope: an atom of the same element but with a different amount of neutrons and therefore different atomic mass.
Electron cloud: the region around the nucleus where electrons are found
What is a polyatomic ion?
An ion made of 2 or more atoms bonded together, usually with a charge
ex. OH-
What are Van der Waals forces?
Weak intermolecular forces arising from temporary shifts in electron distribution
In other words, it is a weak interaction/attraction between atoms that are very close together
What is the difference between a Lewis Structure and a structural formula?
A Lewis Structure shows the pairs of valence electrons and the bonds within a molecule, whereas a structural formula only shows the bonds. Therefore, the Lewis Structure is more detailed.