Has a mass of 1 amu, found in the nucleus, and has a +1 charge
What is a proton
The atomic number tells you what about an element?
The number of protons (which is equal to the number of electrons, when its neutral)
An atom with more electrons than protons, causing it to have a negative net charge.
What is an anion?
The dots represent these on an Electron Dot Diagram.
What are valence electrons?
Protons, neutrons, & electrons
What is a subatomic particle?
In a neutral atom, there are an equal number of these subatomic particles
What are protons and electrons?
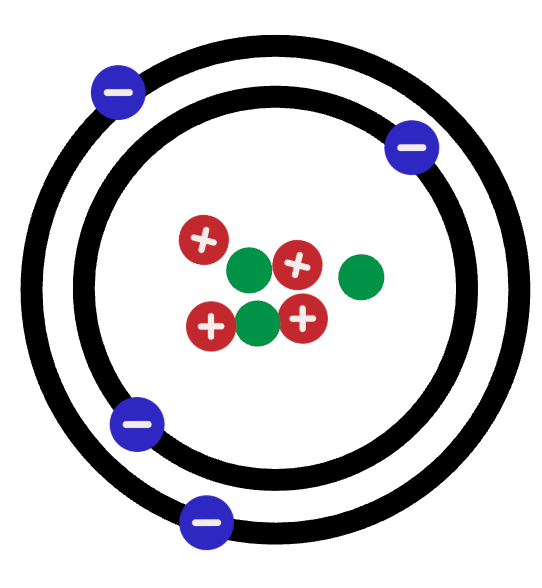
Elements in the same __________ have similar properties.
What are groups?
The net charge of an atom with 5 protons, 6 neutrons, and 6 electrons.
What is -1?
Remember to compare the amount of protons and electrons to find the net charge.
What is the group number?
A horizontal row on the Periodic Table
What is a period?
The amount of protons and neutrons
What is the mass number?
The periodic table is made up of three classes - but most of the periodic table are only 1 class.
What are metals?
If any neutral atom loses 1 electron, it will have this net charge.
What is +1?
The electron dot diagram for Bismuth (Bi).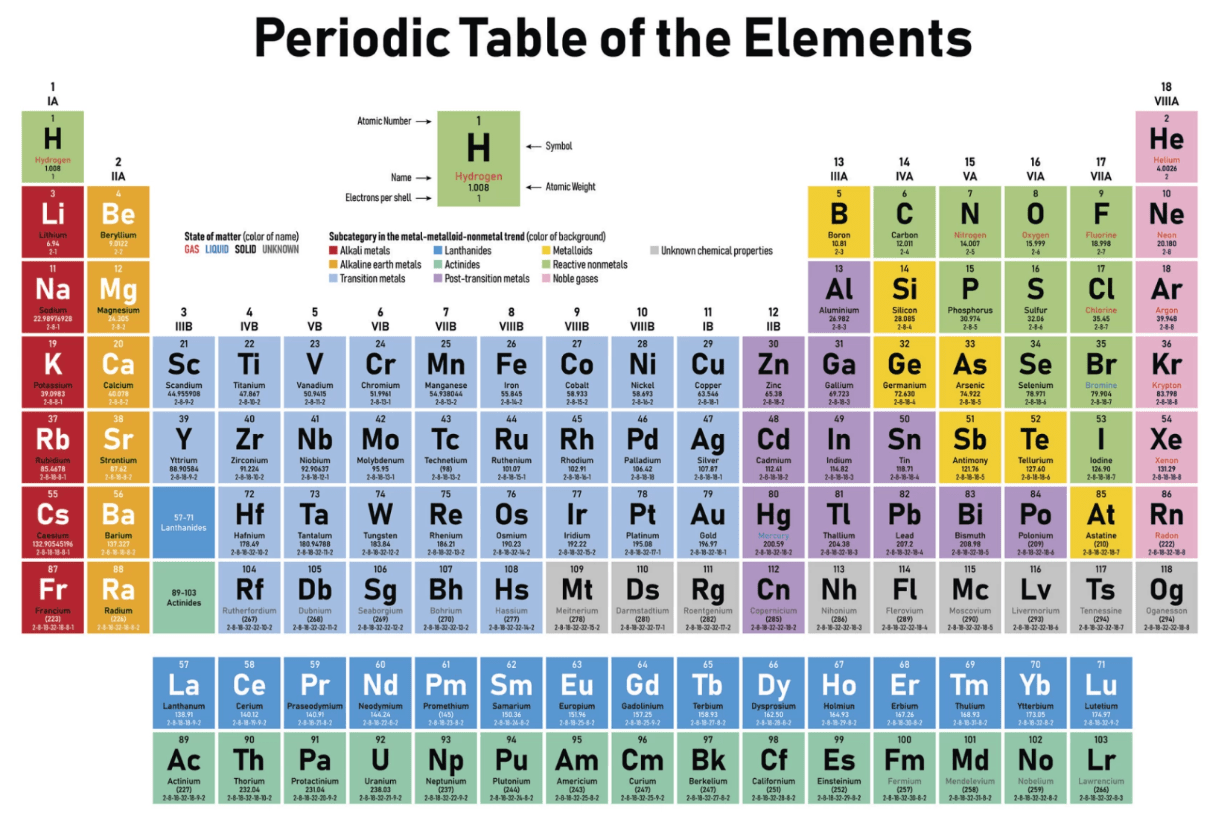
An atom with an unequal amount of protons and electrons, resulting in a net charge.
What is an ion?
2 atoms each have 4 neutrons & 4 electrons, but one atom has 3 protons and the other has 4 protons - these are called ___________.
different elements
The element in period 6 and Group 15.
What is Bismuth (Bi).
Draw an isotope for this atom: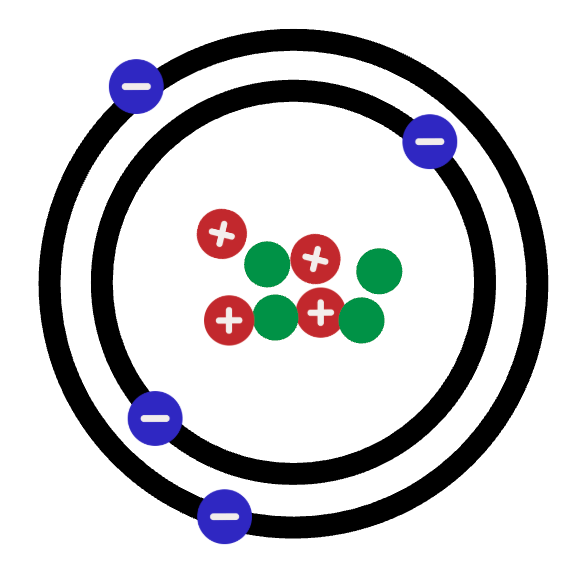
 Or any atom with 4 protons, 4 electrons, and NOT 4 neutrons
Or any atom with 4 protons, 4 electrons, and NOT 4 neutrons
 How many bonds can an atom of Sulfur (S) make?
How many bonds can an atom of Sulfur (S) make?
What is 2 bonds?
Remember to subtract the group number from the number 8!
Atoms of the same element, but with a different mass number.
What are isotopes?
Draw a neutral atom (Bohr model) of Nitrogen (N). 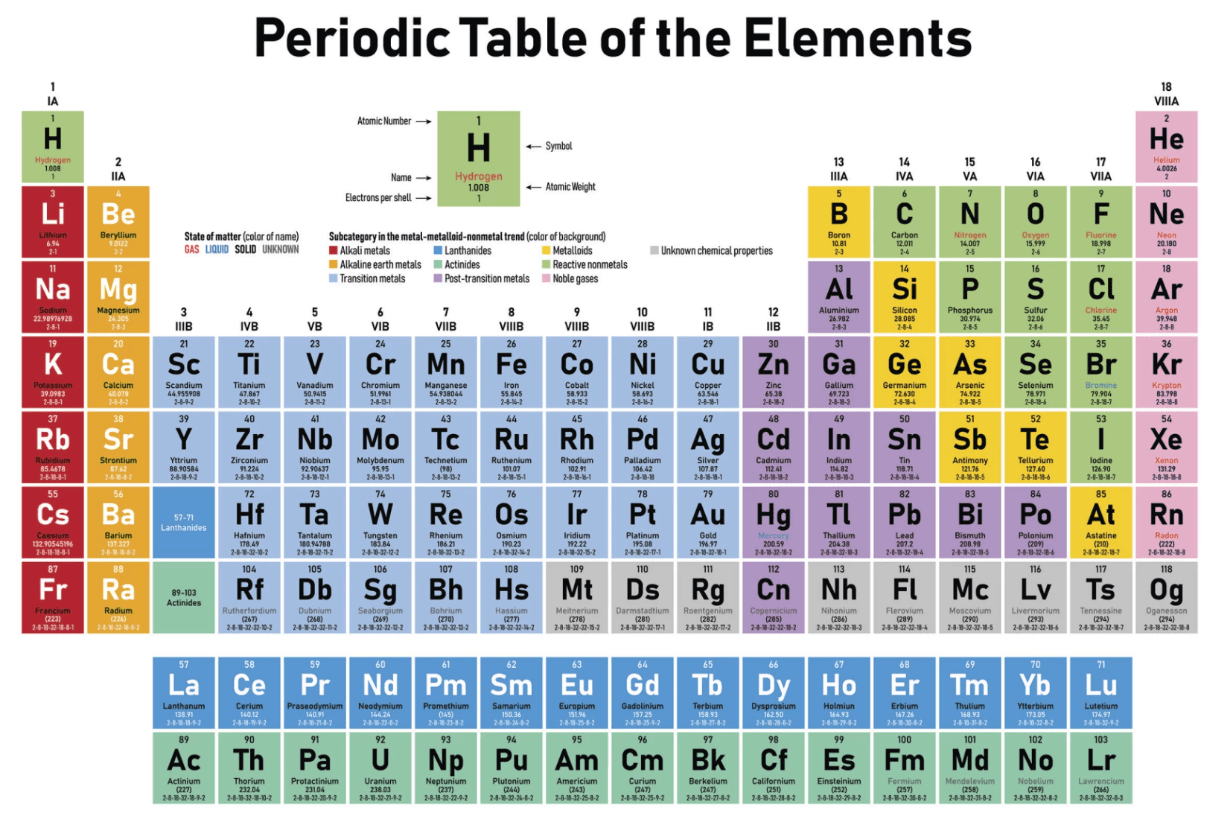
What is:
As you move from left to right ➡️, this trend exists on the periodic table.
What is:
- the atomic number increases
- metals ➡️ metalloids ➡️ nonmetals
Draw a cation for the atom shown: 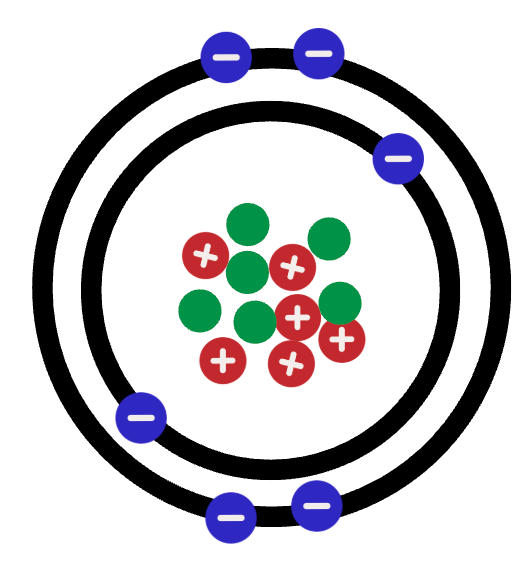
What is: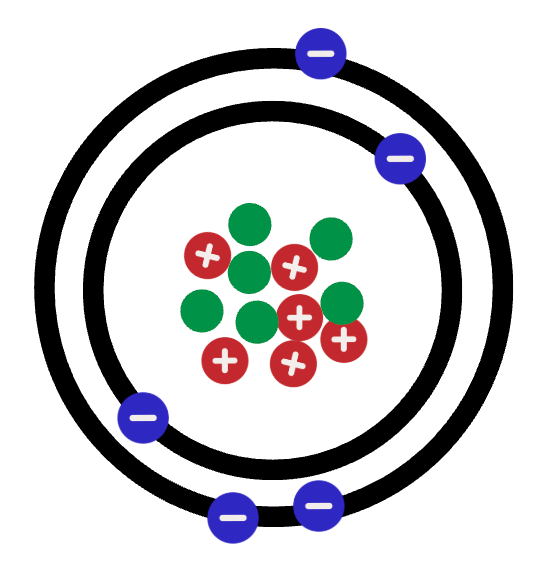 Or anything with 6 protons, 6 neutrons and LESS than 6 electrons
Or anything with 6 protons, 6 neutrons and LESS than 6 electrons
The reason that the dot structure for Helium looks like the image shown instead of having 8 dots.

Helium is in period 1, so it only has 1 orbital. The 1 orbital can only hold up to 2 electrons, not 8. (BONUS POINTS IF YOU NAME THE RULE HELIUM FOLLOWS)
What is the octet rule?