Anything that has mass & occupies space
Matter
Bond that forms when electrons are gained/lost & become charged
Ionic bond
Proton acceptors
Bases
The input of a chemical reaction is the _____ while the result is the ______
Reactant, product
What are carbohydrates?
Groups of molecules that include sugars & starches, provide a ready, easily used source of cellular fuel
Smallest particles of an element, give each element their particular physical & chemical properties
Atom
Bond that forms when 2+ valence shell electrons are shared
Covalent bond
Release hydrogen ions (H+)
Acids
Reactions that involve atoms & molecules combining to form larger, more complex molecules
Synthesis/anabolic reactions
Molecules that are insoluble in water, used for fat storage
Lipids
Name and describe the parts of an atom
Protons: carry a positive charge
Neutrons: have no electrical charge
Electrons: carry a negative charge
An atom that gained 1+ electrons is called a _____ and has a ____ charge
Anion, negative charge
A substance with a pH of 10 is considered
Basic/alkaline
Reactions that involve the breakdown of a molecule into smaller components.
Decomposition/catabolic
Single-stranded molecule used for protein synthesis
RNA
How many neutrons does carbon have?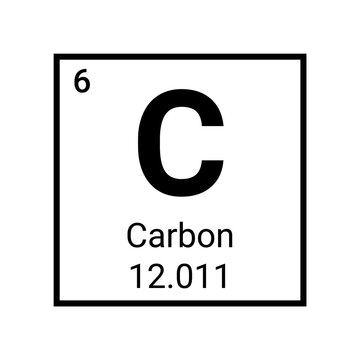
6
Mass number (protons & neutrons)= 12
Atomic number (protons) = 6
12-6 = 6
An atom that lost 1+ electrons is called a _____ and has a ____ charge
Cation, positive charge
Acidic
Reactions that involve both synthesis & decomposition
Exchange/displacement
Monomer: building blocks, single unit
Polymer: many monomers, complex structure
What is an isotope?
Structural variations of the same element, contain the same number of protons but differ in the number of neutrons
Desire to have full electrons in the valence shell
What is a buffer?
Resists abrupt changes in pH by releasing or binding hydrogen ions
Increase rate of reaction without being chemically changed, give a biological example
Catalysts, enzymes
Describe 2 of the 4 structural levels of proteins
1: linear sequence of amino acids
2: alpha helix coils, beta pleated sheets
3: superimposed structure, compact globular molecule
4: 2+ polypeptide chains, complex structure