One or more pairs of shared electrons
Covalent
Mobile valence electrons in a metallic bond that are shared between all the atoms in the bond are called
delocalized electrons
During bonding, atoms want to bond with other atoms to attain a lower energy state to become more stable.
True
Name at least 2 examples of elements which do not obey the octet rule
1. transition metals
2. inner transition metals
3. the first few elements on the P.T.
Name 1 example of a "network covalent substance" that provide exceptions to the normal covalent compound properties.
Diamond or quartz
Molecule with Oxygen and Sulfur
Covalent (nonmetals)
The attraction between opposite charges is called a ______________ force.
electrostatic
The greater the difference in electronegativity between 2 atoms, the lesser the polarity of the resulting molecule after they bond
False
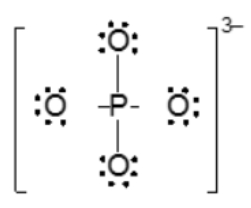
It has gained 3 electrons to help satisfy the octet rule for all the atoms
δ+
What does this mean?
Partial positive charge, used in electronegativity differences between atoms in a molecule.
Molecule with Oxygen and Sodium
Ionic - metal and nonmetal
____________ ions are made up of 2+ atoms and often act as single charged particles in chemical reactions.
Polyatomic
Metallic bonds are characterized by loose transfer of electrons between 2 atoms.
False - electron-sea theory
Name all correct properties for metals using the electron-sea theory:
a. luster
b. conductivity
c. brittleness
d. ductility
A, B, D
The measure of the ability of an atom to attract electrons in a bond is:
a. polarity
b. electronegativity
B. electronegativity
Hard, brittle crystals
high melting points
lusters
Covalent
Mixture of atoms of a metal bonding with another element
Alloys
A chemical's molecular shape determines its function, ability to help medicine, overall properties.
True
Every line or dash in a Lewis structure represents what
2 electrons
Name all options that are properties of ionic compounds:
a. solids have high melting points
b. solids are good heat conductors
c. solids easily split or cleaved
d. liquids are good electrical conductors
A, C, D
ΔEN (or the difference in electronegativity) present within this bond might easily be above 3.0
Ionic - very large electronegativity differences between atoms in a bond
Ratio of cations to anions in an ionic compound is expressed in a chemical formula called a __________ _____
Formula unit
The orderly arrangement of ions in a 3D pattern in a compound is called an ionic network.
False - crystal lattice
Some atoms can share up to how many pairs of electrons?
1 pair (single bond)
2 pairs (double bond)
3 pairs (triple bond)
Give the correct formula unit for a molecule involving Na and O?
Na2O