H2O

(A) Gasoline is dangerous because it easily evaporates to fill a given space with flammable vapors.
or
(B) Carbon monoxide kills by bonding irreversibly to red blood cells.
(SELECT ONE)
lead(II) nitrate → lead(II) oxide + nitrogen dioxide + oxygen gas
a) H2+
b) O2+
c) Mg2+
CO2
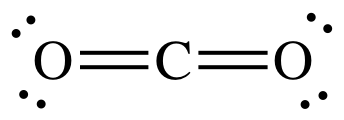
carbon dioxide + carbon → carbon monoxide
CO2 + C → 2CO
(SYNTH rxn)
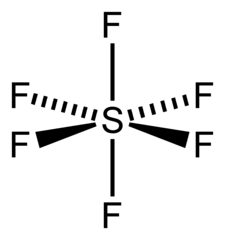
SF6
HNO3 + Al(OH)3 → Al(NO3)3 + H2O
3HNO3 + Al(OH)3 → Al(NO3)3 + 3H2O
(DOUBLE DISPLACEMENT rxn)

copper + silver(I) nitrate → copper(II) nitrate + silver
_
Nitrate = NO3—
Cu + 2AgNO3 → Cu(NO3)2 + 2Ag
(SINGLE DISPLACEMENT rxn)


lead(II) nitrate → lead(II) oxide + nitrogen dioxide + oxygen gas
_
Nitrate = NO3—
2Pb(NO3)2 → 2PbO + 4NO2 + O2
(DECOMPOSITION rxn)