What is brittle/ness?
You can “float” a paperclip on water if you very carefully lay it flat on top of the water. This is due to what is known as _________.
What is surface tension?
What is N2F4?
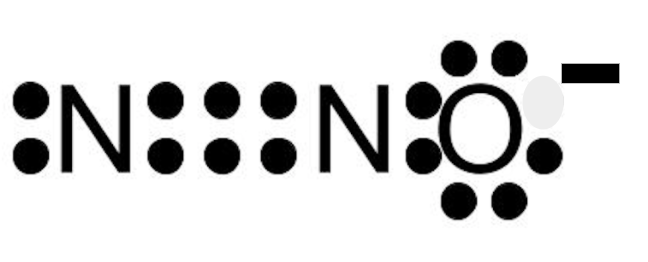
Calculate the molar mass for H2O.
What is 18.0148g/mole?
This is the chemical formula for phosphorous trichloride.
What is PCl3?
Draw the Lewis Dot Diagram for K2O.

Calculate the molar mass of NaNO3.
What is 84.994g/mole?
This is the name for the formula AgNO3.
What is silver nitrate?
Polypropylene is only made from one monomer, which makes it one of these.
What is a homopolymer?
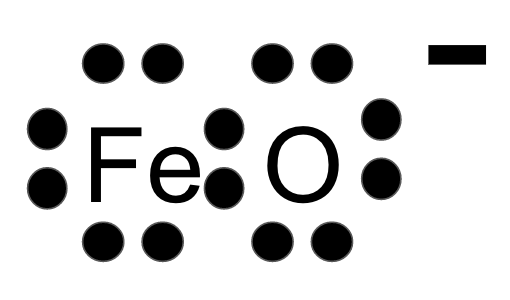
Draw the Lewis Dot Diagram for FeO.
Calculate the molar mass of (NH4)2S?
What is 68.1422g/mole?
This is the chemical name for the formula CuSO4.
What is copper sulfate?