Columns (up and down) within the periodic table are called _____
Families
What are three signs that a chemical change has occurred?
- Produce Light
- Produce heat
-Produce an odor
- Change in color
- Create a precipitate
List the three phases of matter in order from least kinetic energy to most kinetic energy
Solid, Liquid, Gas
Give an example of an alkaline earth metal
Be, Mg, Ca, Sr, Ba, Ra
If atoms give and take away electrons, which type of bond is being studied?
Ionic
metals
nonmetals
hydrogen
oxygen
A _____ is a positively charged particle in the nucleus that determines the element's identity.
Proton
Provide 2 examples of physical change and 2 examples of chemical changes
Multiple answers
A physical change will change shape or size without creating a new substance
A chemical change will create new substances
Presented below is a shower thought. Pick a stance and defend your point:
If you are waiting on a waiter, aren't you the waiter?
HAPPY SUMMER!!! ENJOY THESE 200 POINTS FOR FREE!!!
Give an example of an element with 4 VE's
C, Si, Ge, Sn, Pb
What is the ultimate goal when atoms form a bond?
To reach 8 valence electrons, either by filling or emptying their outer shell.
Balance the following chemical equation:
__ BaCl2 + __ H2SO4 = __BaSO4 + __HCl
What are the reactants?
How many chlorine atoms are on the right side before balancing?
1, 1, 1, 2
BaCl2 + H2SO4
1 chlorine atom
A _____ is a pure substance made from two or more elements chemically combined in fixed proportions.
Compound
A mystery substance has a mass of 171g and a volume of 15 cm3. Based on the table provided, what substance is being observed?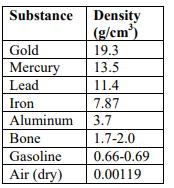
Lead
Answer the following questions from this graph:
- How long is this substance a solid?
- At what letter does the substance have the most kinetic energy?
- At what temperature is this substance finished melting?
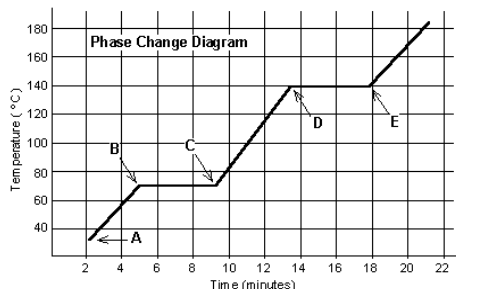
3 minutes
E
70 degrees Celsius
Why does most of the mass of an atom exist in the nucleus?
Protons and neutrons are x2000 larger than electrons, and both of those subatomic particles are located in the nucleus.
Draw the bond for the following elements: Mg and F
Then answer these questions:
- How many electrons does magnesium give up?
- How many fluroine atoms are in your final drawing?
- What is the charge on a single fluroine atom?
Mg gives up 2 electrons
2 Fl atoms are in the final drawing
Fl has a charge of -1
Balance the following chemical equation:
__ Fe + __Cu2O --> __Fe2O3 + __ Cu
What are the reactants?
How many copper atoms are on the left side after the equation is balanced?
2, 3, 1, 6
Fe + Cu2O
6 copper
_____ are atoms of the same element that have the same number of protons but different numbers of neutrons.
Isotopes
Identify the following matter as a pure substance or a mixture.
Then, identify it as an element, compound, homogeneous mixture, or heterogeneous mixture:
Mixture
Homogeneous Mixture
If 22.5 L of nitrogen at 748 mm Hg are compressed to 725 mm Hg at constant temperature. What is the new volume?
Round your answer to the nearest tenths place
23.2 L
Rank the following elements from most ionization energy to least ionization energy:
Selenium (Se), Oxygen (O), Tellerium (Te), Sulfur (S)
O, S, Se, Te
Draw the bond for the following compound: P2
Then answer these questions:
- How many total electrons are needed in your drawing?
- How many double bonds are present in your drawing?
- How many free electrons does your drawing have?
10 electrons
TRICK QUESTION...it's a triple bond!!!
4 free electrons
Balance the following chemical equation:
__Mn(OH)2 + __ HPO3 --> __ Mn(PO3)2 + __ H2O
What are the products?
How many hydrogen are on the right side after balancing?
1, 2, 1, 2
Mn(PO3)2 + H2O
4 hydrogens
_____ is the process where a solid changes directly into a gas without passing through the liquid phase.
Sublimation
Identify the following matter as a pure substance or a mixture.
Then, identify it as an element, compound, homogeneous mixture, or heterogeneous mixture:
Copper Sulfate (CuSO4)
Pure substance
Compound
A gas is heated from 263.0 K to 298.0 K and the volume is increased from 24.0 liters to 35.0 liters by moving a large piston within a cylinder. If the original pressure was 1.00 atm, what would the final pressure be?
Round your answer to the nearest hundredths
0.78 atm
Why does electronegativity increase as you move right across a period in the periodic table?
Why does electronegativity increase as you move right across a period in the periodic table?
Draw the bond for the following elements: Al and O
Then answer these questions:
- How many electrons does oxygen give up?
- How many oxygen atoms are in your final drawing?
- What is the charge on a single aluminum atom?
TRICK QUESTION...none
3 oxygen atoms are needed
Al has a charge of +3
Balance the following chemical equation:
__ Al4C3 + __ H2O --> __Al(OH)3 + __ CH4
What are the products?
How many hydrogen are present on the right side before balancing?
1, 12, 4, 3
Al(OH)3 + CH4
7 hydrogen
_____ is the average kinetic energy of the particles that make up a substance.
Temperature
A mystery substance has the dimensions seen below and a density of 1.88 g/cm3. What is the mass of this substance in grams?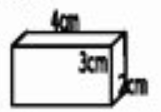
45.12 g
Maybelline Cousteau’s backup oxygen tank reads 900 mmHg while on her boat, where the temperature is 27 degrees celsius. When she dives down to the bottom of an unexplored methane lake on a recently-discovered moon of Neptune, the temperature will drop down to –183 degrees celsius. What will the pressure in her backup tank be in atm at that temperature?
0.36 atm
Fill in the 4 blanks with the correct numbers. You have 3 minutes
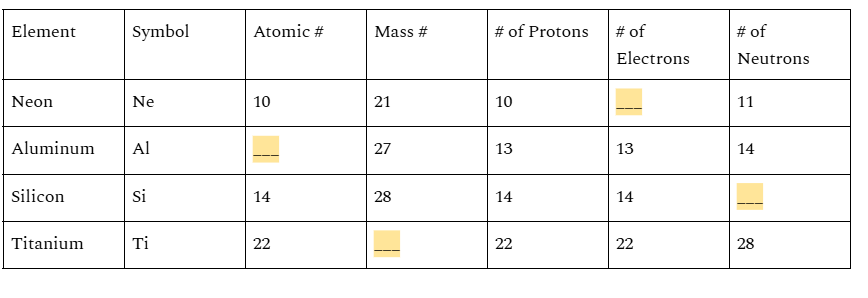
Blank 1= 10
Blank 2= 13
Blank 3= 14
Blank 4= 50
Draw the bond for the following compound: NOCl
Then answer these questions:
- Which element goes in the center of the drawing?
- How many double bonds are present in your drawing?
- Who is happy after initially placing your electrons but before the drawing is complete?
- Nitrogen is in the center
- 1 double bond is present
- Oxygen and Chlorine are satisfied before moving anything around
Balance the following chemical equation:
__ Fe2S3 + __ O2 = __ SO2 + __ Fe2O3
What are the reactants?
How many oyxgen are on the right side before balancing?
2, 9, 6, 2
Fe2S3 + O2
5 oxygens