A particle is a very small piece. About how many times smaller is a particle than the smallest grain of sand?
About 10,000 times smaller
What does the law of conservation of mass say?
Mass cannot be created or destroyed.
Which property tells how much space an object takes up?
Volume
What does it mean to combine substances?
To mix two or more materials together
A type of mixture where one substance dissolves in another substance?
Solution
What is matter?
Anything that has mass and takes up space.
A student mixes baking soda and vinegar in a sealed bag. The bag inflates. What happens to the mass?
The mass stays the same
Name two properties of matter and describe them.
Example: Color describes how an object looks; texture describes how it feels
Other examples: shape, mass, hardness, magnetism, solubility
Which example shows a chemical reaction?
A. Mixing sand and water
B. Stirring sugar into water
C. Mixing baking soda and vinegar
D. Cutting paper in half
C. Mixing baking soda and vinegar
The process that scientists use to explore observations and answer questions?
Scientific Inquiry Process
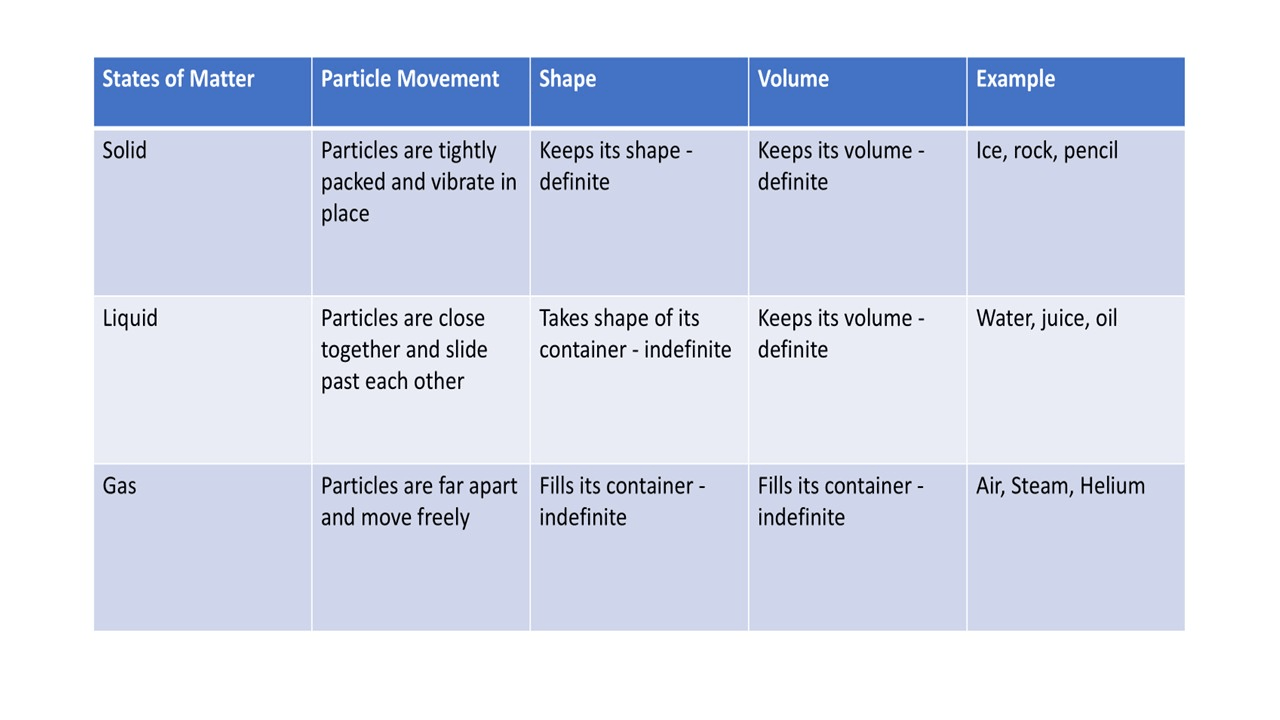
A __________ has particles that are tightly packed together.
A solid
Explain why the mass may appear to change during a reaction in an open container.
In an open container, gases can escape into the air, making it seem like mass was lost.
Why are properties of matter useful to scientists?
Properties of matter help scientists identify, compare, and classify matter.
When two substances are combined and no new substance forms, it is called a:
Physical change
Anything that takes up space and has weight?
Matter
A student heats a cup of water.
What happens to the particles in the water?
How does this help explain why the water might eventually turn into gas?
Heating causes particles to move faster. As the particles gain energy, they spread farther apart, which can cause the water to change into a gas.
True or False
Conservation of mass applies to physical and chemical changes.
True
Explain how mass and volume are different.
Mass is the amount of matter in an object, while volume is the amount of space it takes up.
1. A change where a new substance is formed?
2. A change in size, shape, or state?
3. Matter with particles that move freely?
4. A clue that a reaction occurred?
1. Chemical reaction
2. Physical reaction
3. Gas
4. Evidence
This property of matter describes whether a substance can dissolve in a solvent such as water.
Solubility
Fill in the blanks
Which tool is best used to measure mass?
Balance or scale
Two objects look the same but one feels heavier.
Which property is different?
How could you measure it?
The different property is density (or mass for the same volume), meaning one object packs more matter into the same space. (solid metal ball vs. a hollow plastic ball of the same size).
You can measure this difference using a balance scale (to compare mass)
Explain the difference between a physical change and a chemical reaction.
Describe two signs that show a chemical reaction may have occurred.
A physical change does not create a new substance, while a chemical reaction does.
Examples include gas bubbles, color change, temperature change, or forming a solid.
How heavy something is for its size, or how tightly packed its "stuff" (mass) is in the space it takes up (volume).
Density