Problems
Problems
How does the image show conservation of matter?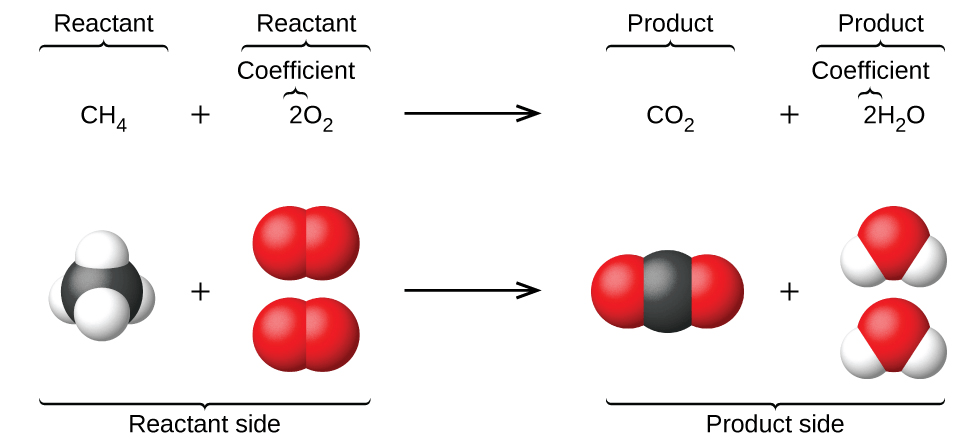
What is the term for the ratio between the coefficients of any two reactants or products in a balanced chemical equation?
mole - mole ratio
4Na(s) + O2 (g) → 2 Na2O(s)
6.50 grams of aluminum reacts with an excess of oxygen. How many grams of aluminum oxide are formed? 4Al + 3O2 --> 2Al2O3
12.3 g Al2O3
What is another name for limiting reagents?
limiting reactants
What is the formula for percent yield?
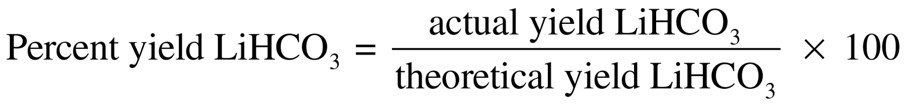
What do the coefficients in a balanced equation tell us?
the number of moles present for each substance
How can you tell when something is going to be a mole ratio problem?
When the moles of one substance is given, and the question asks for the moles of any other substance in the chemical equation/reaction.
ex. 2C2H2 + 5O2 --> 4CO2 + 2H2O
(Bonus!!)
If 10.1 g of Fe are added to a solution of Copper (II) Sulfate, how many grams of solid copper would form? 2Fe + 3CuSO4 --> Fe2(SO4)3 + 3Cu
17.2g Cu
After you have completed your two equations when doing a limiting reagent problem, how can you tell which is the limiting reagent?
the reactant that produces the smallest amount of product
(Bonus!!) The theoretical yield in this reaction is determined by which reactant and why?:
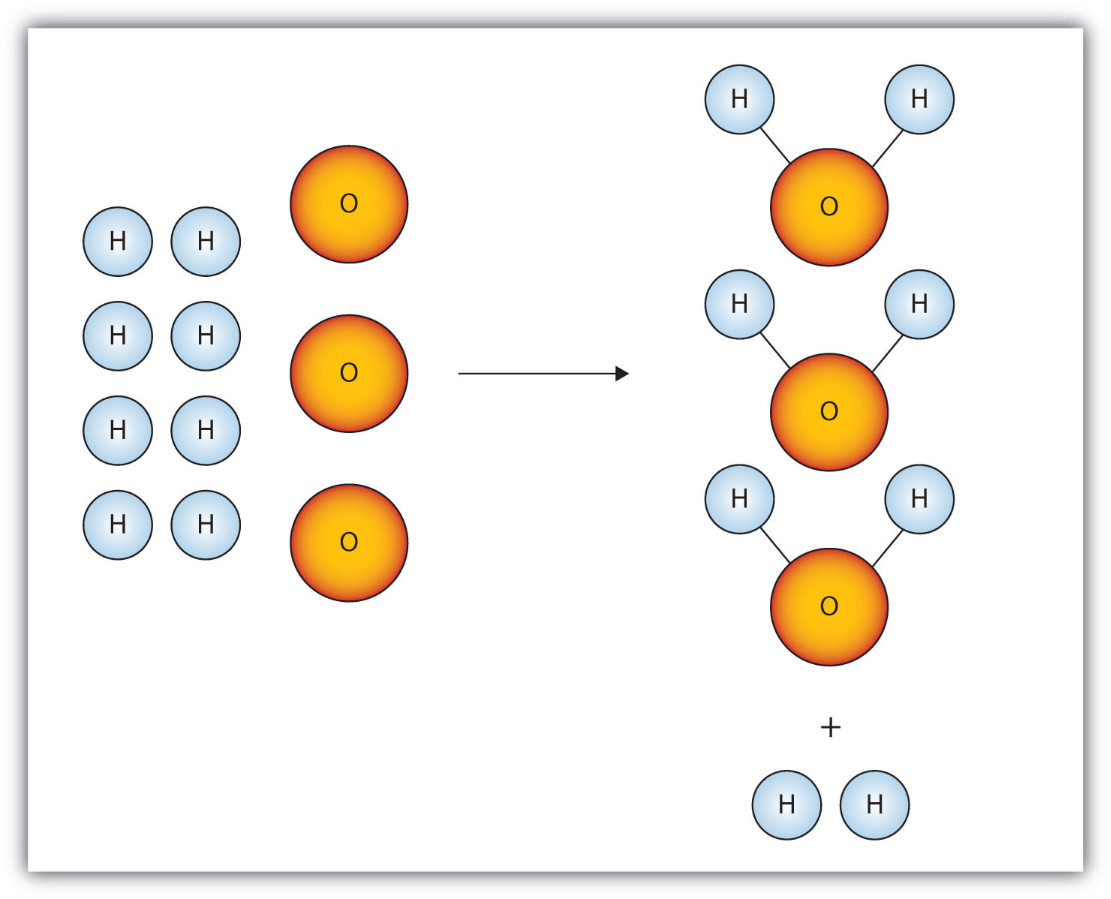
Oxygen, because it is the limiting reactant.
Stoichiometry must always start with a ________________ chemical equation.
balanced
(Bonus!!)
If 3.0 moles Al2O3 decompose, how many moles of oxygen are produced? 2Al2O3 -> 4Al + 3O2
4.5 moles O2
In the reaction of nitrogen gas, N2, with hydrogen gas, H2, to form ammonia gas, NH3, how many moles of hydrogen are needed to react with 1.22 mol of nitrogen?
N2 + 3H2 --> 2NH3
3.66 mol H2
What is a limiting reactant? (What does it do?)
A limiting reactant in a chemical reaction is the substance that -Is used up first -Stops the reaction -Limits the amount of product that can form
What are two ways that your percent yield could be less than 100%?
•Incomplete Reaction:
–some reactant does not react.
•Competing reactions:
–other reactions “steal” reactants limiting the amount of product
•Errors in processing/measurement:
–Some product remains in the source container
–Human error in reading measurements
Bonus!!! (Double Points)
Balance the following chemical equation:
__Fe2O3(s) + __C(s) → __CO2(g)+ __Fe(s)
2Fe2O3(s) + 3C(s) → 3CO2(g)+ 4Fe(s)
Bonus!!
If 3.6 moles of C2H2 are burned, how many moles of CO2 are produced? 2C2H2 + 5O2 --> 4CO2 + 2H2O
7.2 moles CO2
(Bonus!!)
___Mg3N2 (s) + ___ H2O(l) → ___ Mg(OH)2 (s) + ___ NH3 (g)
When 2.00 mol of H2O react, how many grams of NH3 are produced?
Mg3N2 (s) + 6 H2O(l) → 3 Mg(OH)2 (s) + 2 NH3 (g)
11.4 g NH3
When 100.g Mg3N2 reacts with 75.0 g H2O, what is the limiting reactant ?
Mg3N2 (s) + 6 H2O(l) → 3 Mg(OH)2 (s) + 2 NH3 (g)
H2O
Bonus!!
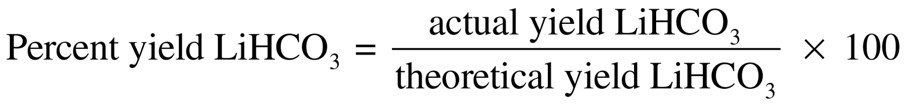
21g of CaCO3 produce 7.2g of CaO, what is the % yield?
CaCO3 --> CaO + CO2
61%
Double Jeopardy!!
Which of the following correctly illustrates the conservation of mass for the reaction below?
4Fe(s) + 3 O2 (g) → 2 Fe2O3 (s)
A) 4.00 g Fe, 3.00 g O2, 2.00 g Fe2O3
B) 223 g Fe, 96.0 g O2, 319 g Fe2O3
C) 40.0 g Fe, 30.00 g O2, 70.0 g Fe2O3
D) 100. g Fe, 100. g O2, 200. g Fe2O3
B) 223 g Fe, 96.0 g O2, 319 g Fe2O3
Bonus!!
2Mg(s) + O2 (g) → 2MgO(s)
How many moles of magnesium are needed to react with 16.0 g of O2?
1.00 mol Mg
Bonus!!
Find the mass of AlCl3 that is produced when 25.0 grams of Al2O3 react with HCl according to the following balanced equation.
Al2O3 (s) + 6HCl(aq) → 2 AlCl3 (aq) + 3 H2O(l)
65.4 g AlCl3
(Bonus!!)
50g of Al are added to 100.g HCl, how many grams of H2 are produced? 2Al + 6HCl --> 2AlCl3 + 3H2
2.74g H2
Bonus!!!
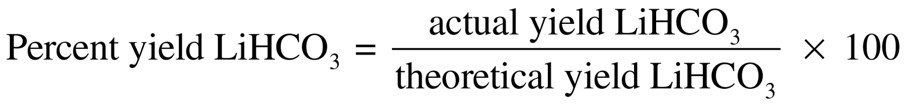
4 Al + 3 O2 --> 2 Al2O3
Calculate the mass of Al2O3 that can be produced if the reaction of 50.0 g of aluminum and sufficient oxygen has a 75.0 % yield.
70.9 g Al2O3