What is temperature related to? (HINT: We learned about this type of energy in Unit 1)
Average Kinetic Energy
Siria receives the following assignments scores in Science:
98 , 98 , 98 , 98
What is her average in Science?
98. Anytime you have the same number, the average is always the same. You do not have to waste time calculating it.
How many seconds did it take for Sample B to reach 100kJ of Thermal Energy?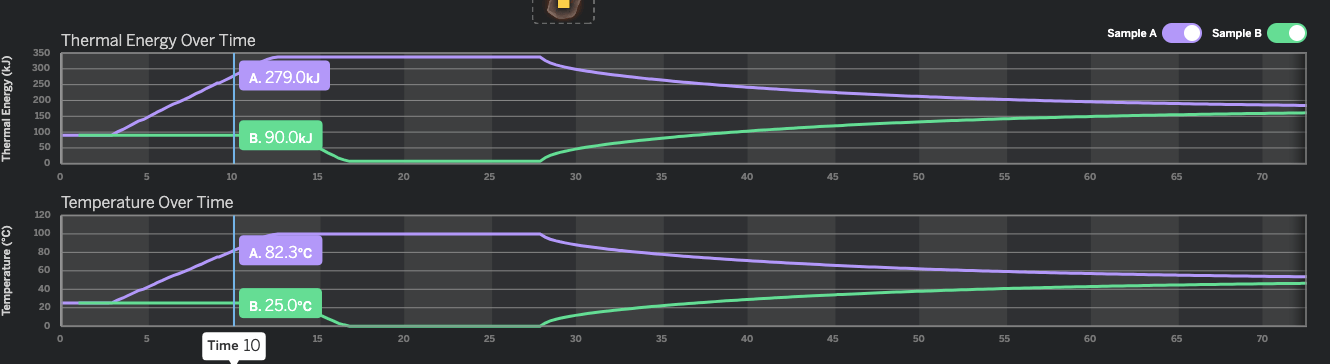
40 seconds
You look at the thermal energy graph. Then look for 100 kJ on the vertical Axis scale. Then see where the green line is (Sample B is the green one) and you will see the green line meets 100 kJ on the vertical axis at about 40 seconds.
How do molecules transfer kinetic energy and change the temperatures of other molecules?
Collisions
Yesterday was a hot day. Describe the molecules of the air.
The molecules were moving fast.
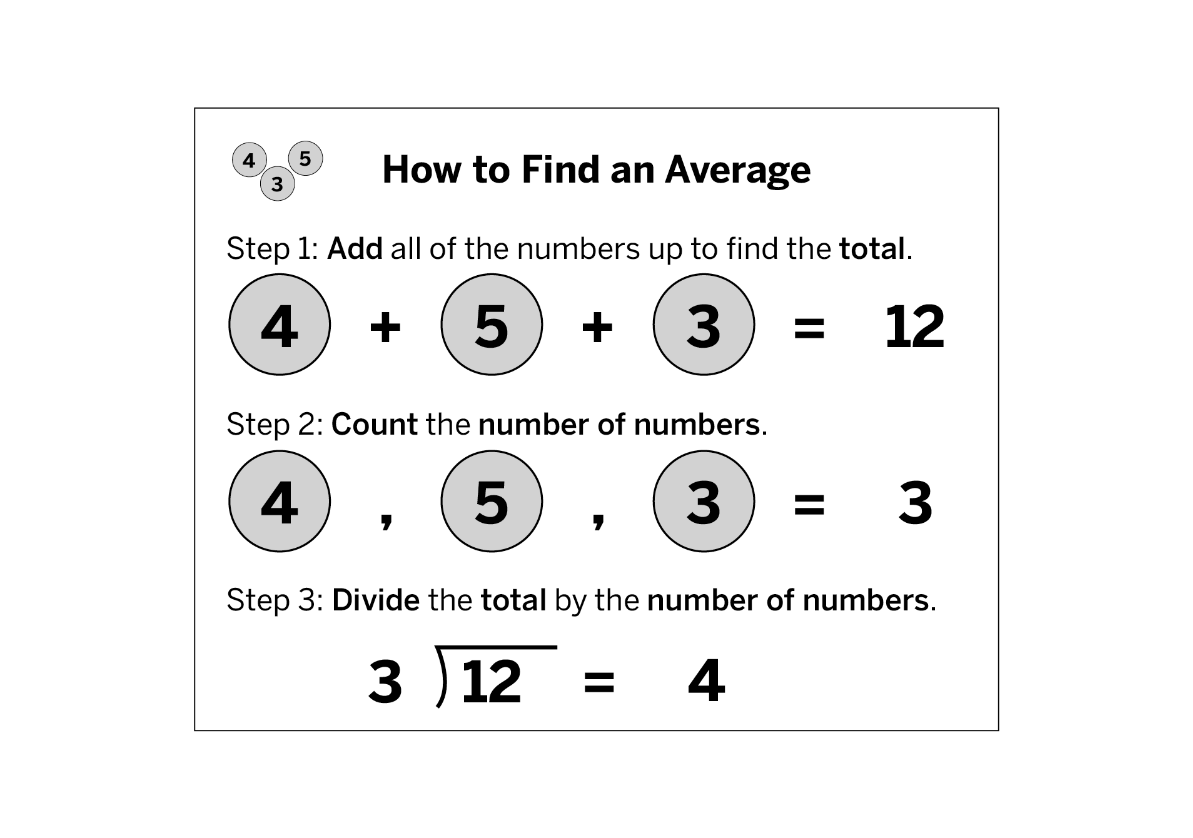
Explain the three steps of calculating an average.
Which statement is most true?
A) I would use a bar graph to show temperature over time.
B) I would use a line graph to show temperature over time.
C) I can not use more than one line in a line graph.
D) Mr. Barone is the flyest guy in BK Studio.
B) I would use a line graph to show temperature over time.
The starting temperatures of two samples are:
SAMPLE A: 30℃
SAMPLE B: 20℃
After putting them in contact, what would be their ending temperature?
25℃
The ending temperature is always halfway between, or the exact middle of the two starting temperatures. This exact middle can also be calculated by finding the average.
Which sample is hotter?
BONUS QUESTION: How do you know?
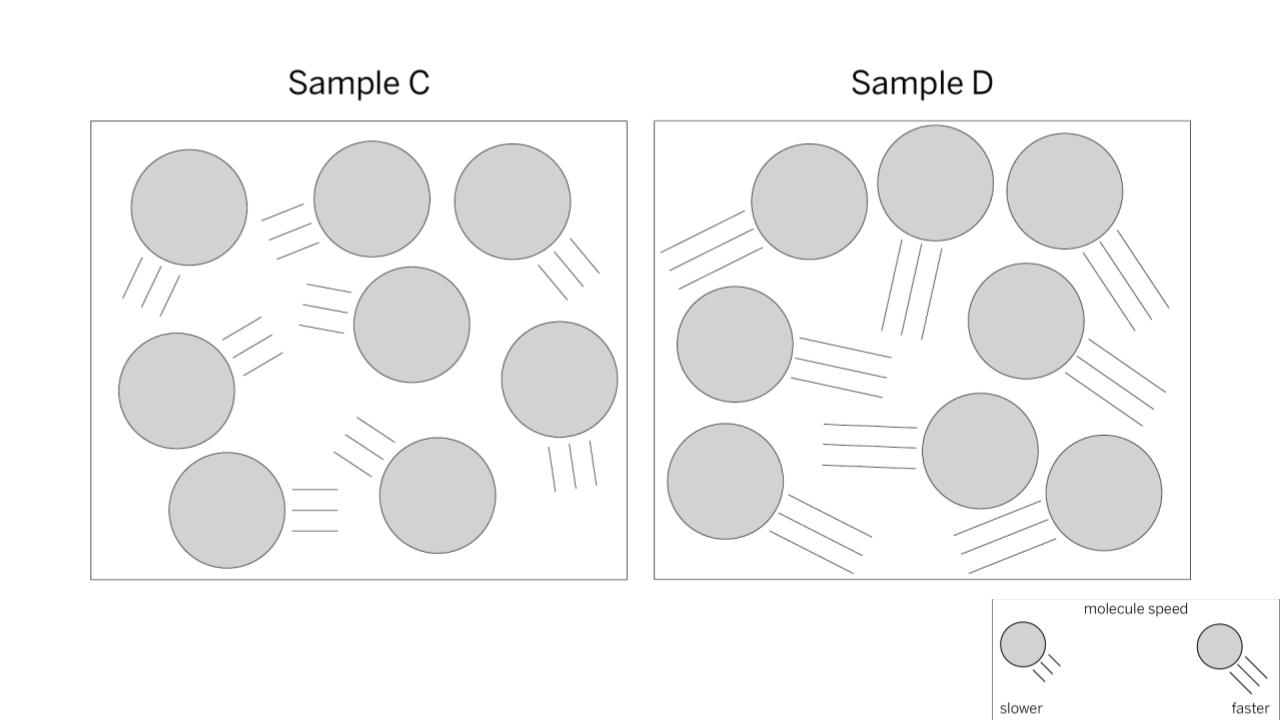
Sample D. Hot temperatures means that molecules are moving faster. The model shows faster moving molecules with longer tails. Sample D shows molecules with longer tails.
Maylin observes the following amount of squirrels come to the BK Studio grass each day.
Mon- 2
Tues - 3
Wed - 3
Thur - 5
Fri - 2
On average, how many squirrels come to the BK Studio grass?
STEP 1:
2+3+3+5+2 = 15
STEP 2: 5 days
STEP 3: 15 / 5 days = 3 squirrels per day
What is the approximate temperature of Sample A at 6 seconds?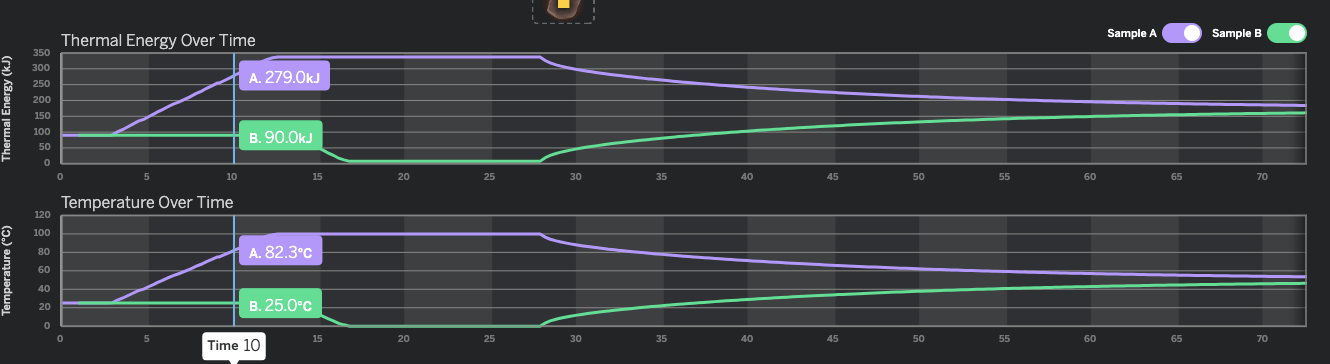
50℃
We are looking at the temperature graph, and the purple line (Sample A). 6 seconds is between 5 and 10 seconds on the horizontal axis, but closer to 5. If we go up the the purple line from 6 seconds, we see it intersects at about 50℃.
TRUE OR FALSE: When two samples come in contact and reach the same temperature, their molecules stop moving.
FALSE: They do not stop moving, they just move at the same speed. If they stopped moving, this would be absolute zero.
Which images correctly display a hot and cold sample?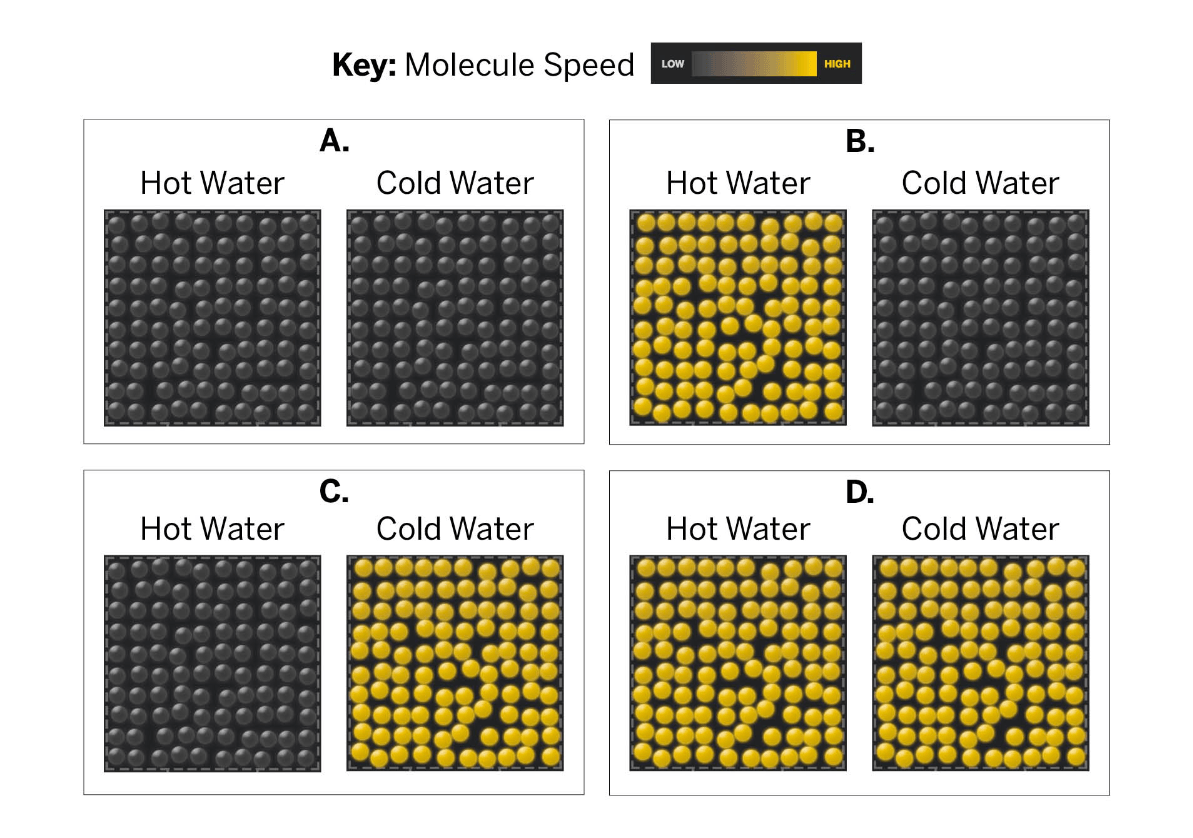
Letter B.
Faster moving molecules are shown in bright yellow, similar to our simulation. Hot temperatures means molecules are moving faster. Letter B is the only one to show fast moving molecules (yellow) for the hot water and slow moving molecules for the cold water.
Which sample is hotter? You must explain to get the points.
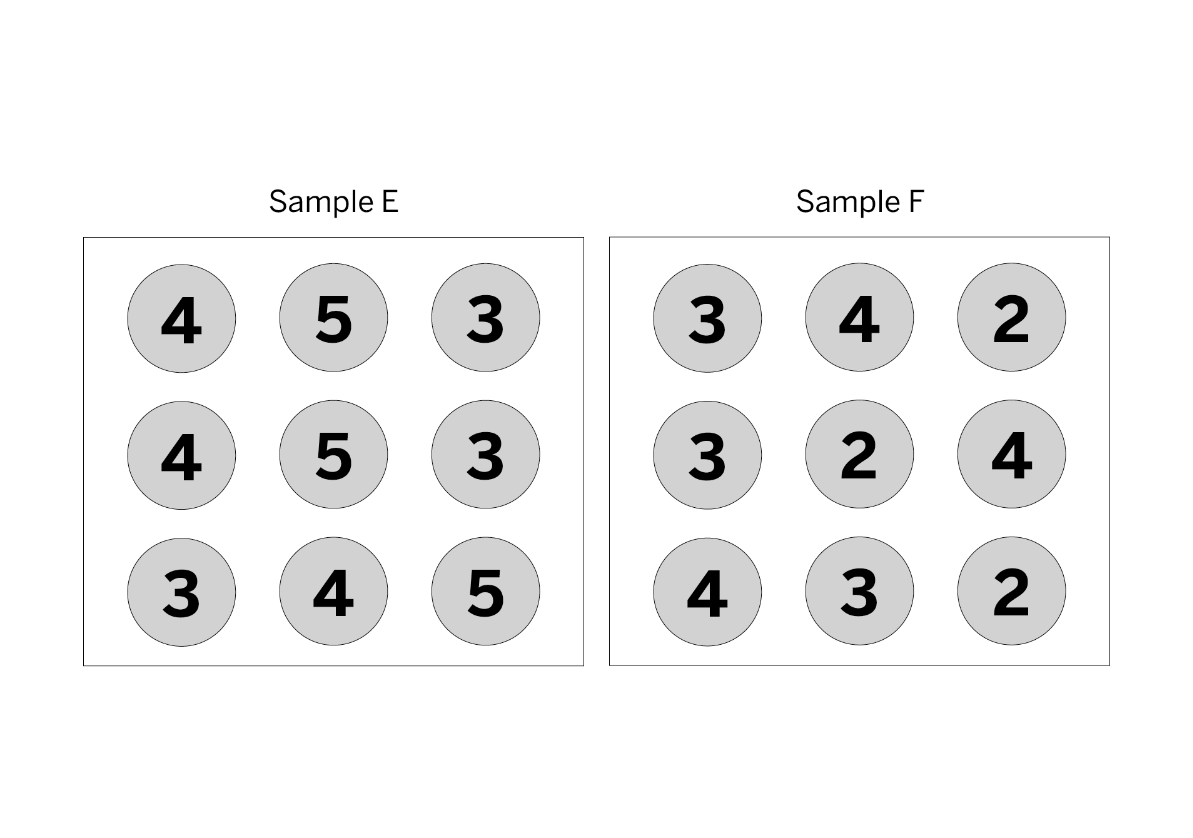
Sample E because it has a higher average kinetic energy.
You would find the average kinetic energy of both samples. The one with the higher average kinetic energy has a higher temperature.
Sample E
STEP 1: 4+5+3+4+5+3+3+4+5= 36
STEP 2: 9 molecules
STEP 3: 36 / 9 molecules = Average KE of 4
Sample F
STEP 1: 3+4+2+3+2+4+4+3+2= 27
STEP 2: 9 molecules
STEP 3: 27 / 9 molecules = Average KE of 3
4 is more than 3
At approximately how many seconds do the two samples stop transferring energy?
Bonus Question: Why?
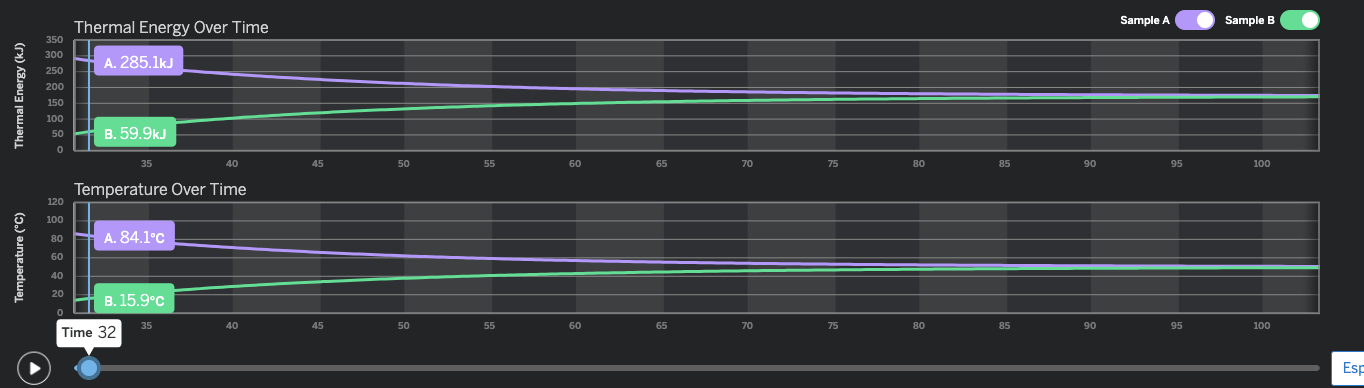
Around 95 seconds.
Do molecules ever stop moving?
If they did, it would be called absolute zero, but so far, it has never happened, even though scientists in labs have come close.
Explain why food dye would spread slower in a cold water sample than a hot water sample.
USE THESES WORDS:
KINETIC ENERGY
MOLECULE
TEMPERATURE
The molecules of the water with a higher temperature move faster, or, have more kinetic energy. Therefore, they would spread the food dye around faster.
Which sample is hotter? You must explain to get the points.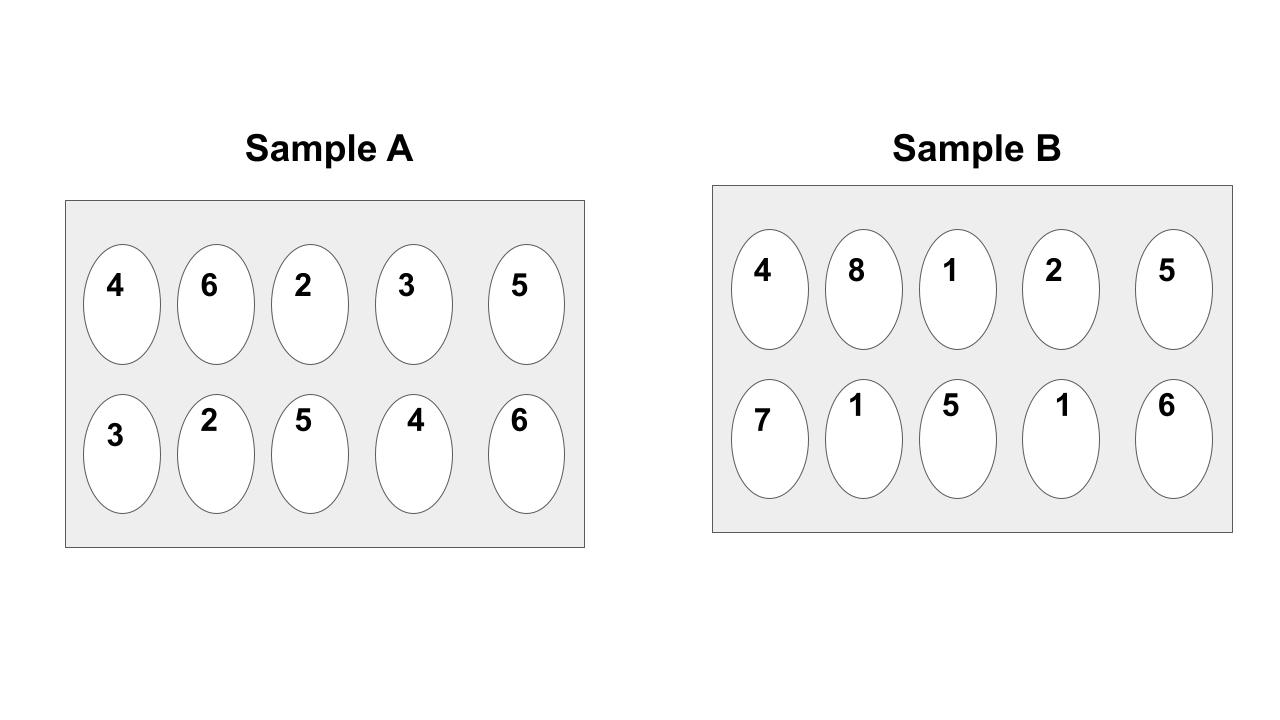
They are both the same temperature because they have the same average kinetic energy.
At approximately how many seconds do the two samples come in contact?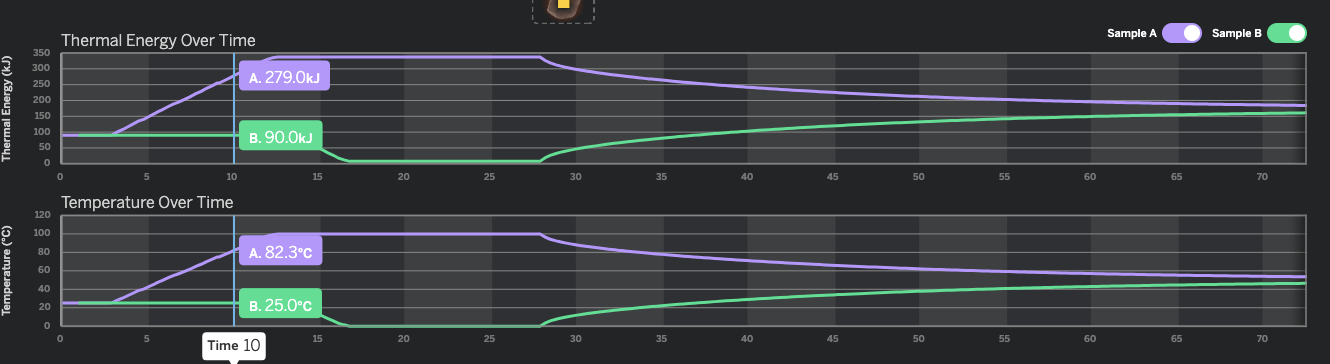
Around 27 seconds
Draw what is happening when the heater heats up the molecules of air.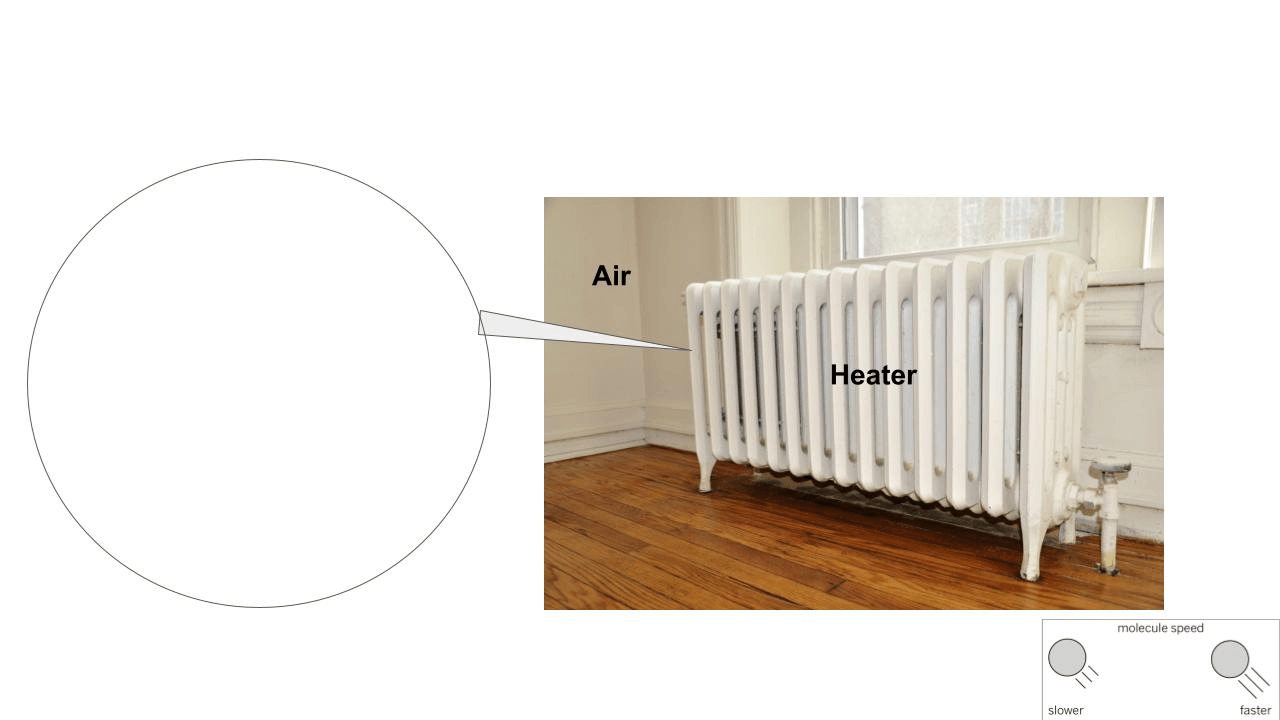
The zoom in on the image should show faster molecules colliding into slower molecules.